Secondary Prevention of Cervical Cancer
Virus Binding and Cell Entry
Virus Binding and Cell Entry
How does HPV infect the cell?
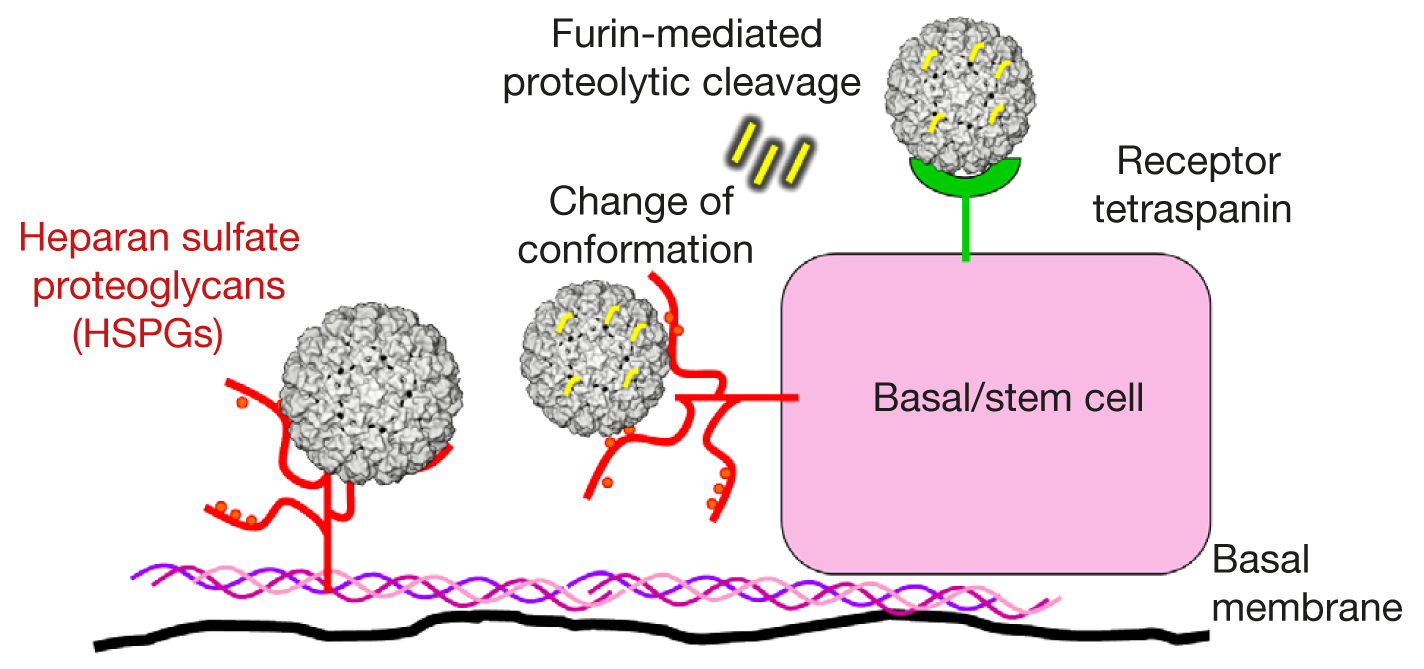
Molecular mechanisms of virus binding and cell entry are now understood. The basal membrane partially comprises of collagen, which has a triple helix structure. The basal membrane and the cell surface are also connected to heparan sulfate proteoglycan (HSPG) molecules that are negatively charged and account for elasticity. HPV attaches to HSPG molecules of the basal membrane or the cell surface. Furin, a protease, is an important enzyme released during wound repair by the dividing cells. In all instances, when stem cells are involved in tissue repair by closing a gap in the basal epithelium layer, furin is released. Furin activity leads to cleavage of the HPV L1 capsid protein and promotes its conformational changes, which in turn, allows the L1 capsid protein to bind to the cellular receptor tetraspanin and opens up a gateway for the virus particle to enter the cell. During this binding process, the HPV L2 capsid protein becomes accessible, which therefore may be a suitable target for future vaccines. Considering, that the entire process of binding and cell entry takes up several days, there should be enough time for vaccine interaction.